
Risk Governance in Medical Device Manufacturing
Governance, as one of the ESG pillars, is linked to corporate transparency, regulatory compliance, risk management, and the quality of decision-making.
For a medical device manufacturer, governance goes beyond regulatory compliance.
At BriteMED, governance serves as the cornerstone for ensuring patient safety, protecting customer brand reputation, and maintaining a stable supply chain.
This is why Risk Management is embedded at the core of our governance framework. Personally supervised by the General Manager, it ensures that every decision balances business growth with social responsibility, ultimately providing customers with trustworthy medical device manufacturing solutions.
1. Comprehensive Risk Management System
Risk management is a fundamental pillar of BriteMED’s governance strategy. Its objectives include:
- Identifying potential risks: regulatory compliance gaps, proactive evaluation of competitor non-compliance events, supply chain disruptions, and cybersecurity threats, etc.
- Assessing risk impact and probability: through quantitative and qualitative analysis to accurately determine risk levels.
- Developing response strategies: ensuring that even under uncontrollable circumstances, risks are transformed into controllable outcomes and damages are minimized.
We follow a strict Risk Management Plan, covering:
- Risk Analysis
- Risk Estimation
- Risk Control
- Residual Risk Evaluation
- Risk Management Review
- Post-market Information Monitoring
By implementing rigorous and structured risk management, BriteMED provides customers with a stronger commitment to safety. We recognize that both social responsibility and brand reputation are the most critical cornerstones of a sustainable medical device manufacturing company.
2. Sustainable Supply Chain Governance
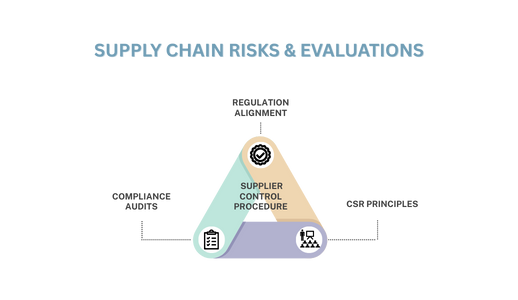
Sustainable Supplier Governance lies at the intersection of ESG governance and environmental/social responsibility, aiming to stabilize the supply chain and monitor accountability while aligning with corporate sustainability goals and regulatory requirements.As a trusted international OEM medical device manufacturer and a leader in contract medical device manufacturing, BriteMED applies Supplier Control Procedure to manage supply chain risks and conduct strict supplier evaluations. We regularly perform compliance audits and establish cooperative frameworks aligned with regulations, CSR principles, anti-corruption policies, and ethical codes, including: .
- Labor rights and human rights protection
- Workplace safety and health management
- Compliance with environmental, occupational safety, and international standards
- Fair trade and anti-corruption practices
One of the core values of sustainable supplier governance is its continuous cycle of contracting, evaluating, improving, and transparent disclosure. This cycle reduces supply chain risk, enhances brand reputation, ensures long-term stability, and meets the sustainability requirements of international clients and regulations (such as EU CSRD, CBAM, and LkSG).
Through these standards, we work closely with suppliers to drive sustainable development, achieving medical devices production goals that support social responsibility, shared prosperity, and ESG in manufacturing.
3. Regulatory Compliance and Quality Management
For over 16 years, BriteMED has specialized in medical device manufacturing. Our Regulatory Compliance Strategy ensures that products remain compliant across research, development, production, market launch, and the full product lifecycle. This includes:
- ISO 13485 (QMS), ISO 14971 (Risk Management), IEC 60601 (Electrical Safety), and IEC 62366 (Usability Engineering)
- Extensive global experience in medical device manufacturing certification and regulatory approvals (from regulatory pathway planning, to design and verification compliance, to post-market surveillance and continuous improvement).
This expertise enables us to adapt flexibly to different market requirements, ensuring that products at every stage—from design and medical device production to commercialization—meet or exceed international medical device manufacturing standards.

4. Cybersecurity Governance and Product/Service Security
In the era of digital and smart healthcare, cybersecurity in medical device manufacturing is critical. BriteMED complies with global information security regulations and follows ISO 27001 standards to establish a strong information security management system, ensuring:
- Reduced cybersecurity and compliance risks
- Improved system resilience and disaster recovery capabilities
- Continuous monitoring and periodic improvements
For product and service safety, we established a Product Security Incident Response Team (PSIRT) and launched a Bug Bounty Program to strengthen product integrity, system security, and user data protection.

5. Business Continuity and Responsibility Commitment
BriteMED conducts annual Business Impact Analysis and Risk Assessment to ensure rapid response and operational continuity in the face of market changes, supply chain challenges, or external shocks.
For us, our customers’ vision is our vision. Through ESG Governance and continuous risk management, we remain committed to society, the environment, and our partners.
